The Role of Mirror Neurons relative to the Core Stuttering Pathology and Compensatory Stuttering Behaviors
Article information
Abstract
Purpose
Persistent developmental stuttering is generally considered to be a speech disorder characterized by repetitions, prolongations and postural fixations that is relatively resistant to therapy. While mainstream stuttering therapy continues to rely on behavioral speech targets, research suggests that mirror neuron networks can be activated to temporarily induce natural sounding fluent speech via exposure to second speech signals. As a consequence, the mirror neuron system model of stuttering predicts that initiating gestural primes would be equally effective at enhancing fluency whether they are produced or perceived by the speaker. This study tests this notion by measuring the effects of producing and perceiving an initiating silent oral opening oral gesture on stuttering frequency.
Methods
Eight participants of varied overt stuttering severity completed one control and four experimental speaking conditions. Participants read aloud 300-syllable passages for all speaking conditions; four experimental speaking conditions tested the effects of endogenously-produced and exogenously-perceived opening oral gestures.
Results
Study data reveal that both the production and perception of initiatory gestural priming significantly enhance fluency. There were no significant differences between the perception and production of the initiating oral opening gestures.
Conclusions
Coupled with existing research, these data suggest a primitive response of the action understanding achieved by mirror neuron networks, thereby enabling an individual who stutters to fluently initiate speech via a primitive lower order network, and bypassing the activation higher order linguistic networks where the neural circuitry associated with the etiology stuttering is speculated to occur.
INTRODUCTION
Persistent developmental stuttering (PDS) is generally considered a speech disorder, and is behaviorally defined as the occurrence of whole-word and part-word repetitions, prolongations, or postural fixations during at least three percent or more during speech production [1,2]. Recent research has provided consistent evidence linking the pathology with genetic and neurological origins [3–10].
Although a cure for stuttering remains elusive, research suggests that enhanced fluency is associated with the gross changes in speech-related neural processing patterns [9,10]. Neurophysiological research has emphasized the importance of motor preparation by highlighting the dysfunctions in sensorimotor programs transmission to the motor cortex, specifically the left hemisphere [10–12]. Several studies in particular report both anatomical and functional disturbances in Broca’s Area (Brodmann area 44, 45) in the left hemisphere [9]. Furthermore, many neuroimaging studies report correlations between functional activation patterns from within the basal ganglia-thalamo-cortical loop and stuttering behaviors [10,13–15].
Numerous reports cite robust fluency enhancement when the person who stutters is exposed to a secondary speech signal (SSS), which is speech feedback of a second concurrent and kinetically similar speech signal received synchronously or asynchronously relative to the primary spoken speech signal [16–18]. These SSS utilize the strong link between the perception of (speech) gestures when matched with the speaker’s targeted (speech) gestural production to enhance the fluency of those who stutter [19,20]. The SSS can be presented exogenously (i.e., externally) through auditory, visual, or tactile sensory modalities [7,19,20]. Additionally, endogenous self-generated primes, such as manual gesturing concurrent with speech production [1,17,20], self-generated gestures such as a silent oral opening frame [1,17,20], or any number of other motoric gestures [1], are also documented as enhancing fluency in those who stutter when paired with the initiation of the (speech) gesture [1,16,18–20]. Research indicates that these speaking conditions may enhance fluency in those who stutter by inhibiting, or bypassing, the higher order neurological block that has been associated with PDS [17,20]
How the multi-sensory SSS enhances fluency has yet to be clearly documented; however, research suggests that the enhancement of fluency by a SSS may be achieved through the activation of the mirror neuron system [7,19,20]. Mirror neurons are primitive neuronal substrates that fire equally when an action is observed as when the same or similar action is performed, thus placing the observer in the same casual state as the actor, creating a strong perception-production correlation [7,10,20–22]. Mirror neurons allow motoric gestures, such as speech, to be immediately recognized and a representation of that action mapped for imitation thus helping to bridge the gap between one agent and another through action understanding [20,23–25].
Research suggests that mirror neurons achieve action understanding by simulating the ‘goal’ of the action, as opposed to simulating the observed action in one’s own motor system [16,21,23]. However, the closer in proximity that the ‘goal’ of the observed action is to the performed gesture, the stronger the perception-production correlation is in for action understanding mirror neurons (AUMN) [7,21,25,26]. Relative to the enhancement of fluent speech in those who stutter, data supports the activation of action understanding relative to the efficacy of fluency enhancement, as the primes that most closely resemble speech gestures have been found to have greater and more robust fluency enhancement [7,21,25,26].
Recent data suggest that the nature of fluency enhancement via gestural primes align with action understanding, thus supporting the mirror neuron system hypothesis as a theoretical model for the enhancement of fluency in those who stutter. Specifically, when manual and oral initiatory gestures were compared relative to their efficacy at fluency enhancement, speech-like oral gestures provided the most robust fluency enhancement [25]. These findings are both predicted and supported by existing research [23,25,27–29]. Likewise, data also suggests mirror neuron involvement relative to fluency enhancement via the perception and production of syllabic repetitions prior to initiating speech production [17]. These data reveal a perception-production link in speech can facilitate the inhibition of stuttering, even among unmatched gestural stimuli; however, the most robust fluency enhancement were achieved through listening to matched repeating syllables [17]. Tangentially, research has documented that both perception and production of manual gesturing results in significant fluency enhancement of those who stutter [20]. While the exact neural mechanism accounting for enhanced fluency remains unknown, the mirror neuron system hypothesis provides a theoretical framework and neural substrate that can account for how a SSS enhances fluency in both production and perception.
Accordingly, as predicted by mirror neuron system hypothesis, research suggests that endogenous gestural primes most closely resembling speech gestures yield the most efficacious fluency enhancement [20,25]. Data also suggest that perception of an exogenous SSS is likely more efficacious at enhancing fluent speech relative to the endogenous production of SSS alone, and that the combination of sensory perception and endogenous production of a stimulus will have greater treatment efficiency through the activation of mirror neuron networks. Therefore, the purpose of this study is to test the effects of perception and/or production of an initiatory gesture (e.g. a silent opening oral gesture) on overt stuttering frequency. If the perception and/or production of a silent opening oral gesture utilizes action understanding achieved by the mirror neuron system, then the model predicts that similar efficacy of fluency enhancement as a function of either production and perception.
METHODS
Participants and study design
Eight adults with PDS (seven males and one female) participated in this research. The age range of research participants was between 18 and 51 years; the mean age of research participants was 30.25 years (SD=9.87, SE=3.49). Four of the participants were informally judged as being mild-moderate in severity; two participants were judged to be moderate in severity, with the remaining two participants being moderate-severe in overt stuttering severity. Given that stuttering behavior is typically defined as three percent or more stuttered syllables (i.e. whole-word and part-word repetitions, prolongations, or inaudible postural fixations) during speech production [1,30], a three percent stuttering frequency in a controlled speaking environment served as an inclusion criteria [7,8,20, 25]. Educationally, one participant successfully earned a high school degree; the remaining 7 participants successfully completed either an Associate’s (2-year) or Bachelor’s (4-year) college degree. Participants were all right-handed dominant, native English speakers who reported normal or corrected vision, and no other diagnosed speech, attention or language disorders. All participants acknowledged understanding, and signed an informed consent form prior to participation in this study.
Protocol
In the control and four experimental speaking conditions, participants were asked to read select passages from a junior high school science textbook, all of which have been used in previous research [7,8,19]. Each passage, consisting of approximately 300 syllables, was divided into 5 to 7 word phrases and was printed on large double-sided cue cards [7,8,20]. For all speaking conditions, each participant sat at a table (approximately 75 cm in height), and was asked to read each phrase aloud. As any novel change in production is known to enhance fluency [1], participants were advised to speak at their normal rate and not to use any previously learned speech techniques that may help alter, control, or reduce stuttering behaviors [7,20]. Both speaking conditions and reading passages were balanced using a Latin Square; this is to say that both the order of speaking conditions and the order of speaking passages were counterbalanced during data collection. All five reading passages were taken from the same text, thereby providing consistent linguistic complexity between passages. A five-minute break was taken between each speaking condition, to help reduce carry-over effects between experimental conditions. This basic research protocol has been used in previous peer reviewed research [7,8,19]
Control and experiment speaking conditions
Each participant completed a control speaking condition and four experimental speaking conditions, which all included a silent oral opening gesture immediately preceding speech production. For the purposes of this study, a silent opening oral gesture was defined as a silent opening mouth gesture as a means to initiate speech production. The silent oral opening gesture (G) provided the core behavioral gesture in which to test the mirror neuron system hypothesis by comparing fluency enhancement resulting from either: (a) self-generated (S) initiatory silent oral opening gesture (G) providing no visual feedback (SG−VF); (b) externally-generated (E) initiatory silent oral opening gesture providing visual feedback (EG +VF); (c) simultaneous production and perception of self-generated silent oral opening gesture providing visual feedback (SG+VF); and (d) simultaneous endogenous production and perception of exogenously generated silent oral opening gesture providing visual feedback (SG+EG+VF). These four experimental conditions approximated different levels of action understanding when paired with the initiation of each initial speech gesture at the beginning of every phrase spoken by the participant.
For the control speaking condition, participants were instructed to read each phrase aloud without the use of a syllabic gestural prime. During the first experimental speaking condition, the participant produced a self-generated initiatory silent oral opening frame (SG−VF) as a means to initiate speech production. A second experimental speaking condition consisted of participant simultaneously producing a self-generated initiatory silent open oral frame and receiving visual feedback from this gesture (SG+VF). This was achieved through the use of an AudiSee (Audisoft Technologies, model #HD-01A-0301-024), which is a head mounted video camera system, providing participants with a 14-centimeter visual display (measured diagonally) focusing on their lips, mouth, and jaw. This visual display was approximately 40 centimeters from the participant at his or her eye level; this visual feedback served to initiate speech production.
Another experimental speaking condition was an externally generated initiatory silent open oral frame gesture with visual feedback (EG +VF). The experimenter wore the AudiSee device and provided the study participant with a silent oral opening frame visual prime which was use to initiate speech. In the final experimental condition, the experimenter again wore the AudiSee device while providing the participant with a visual feedback of a silent oral opening frame. When the participant began to see oral movement on the visual display, they were instructed to produce a silent oral opening frame of their own before starting speech. This final condition was the co-occurrence of a self-generated initiator silent speech gesture with the visual perception of an externally generated initiatory silent open oral gesture (SG + EG +VF). This combination of self-generated initiatory speech with externally generated feedback closely resembles a choral silent gesture. In all experimental speaking conditions, the different levels of action understanding gestural priming immediately preceded, and therefore initiated, speech production.
Data collection and reliability analysis
All participant responses and speaking trials were video recorded using a Hi-8mm video camera (Sony #CCD-TRV75), and a lapel microphone (RS #33-3003) that was attached no more than 15 cm from their mouth with an approximate orientation of 0° azimuth and ~180° altitude. Stuttering syllables were counted from the first 300 syllables of each speaking condition. Moments of overt stuttering were operationally defined as whole- and part-word repetitions, prolongations, or inaudible postural fixations [1,19].
RESULTS
The distribution of stuttering frequency as a function of action understanding gestural priming speaking condition is presented in Figure 1. The mean values of stuttering frequency was 23.13 stuttered syllables (SD=17.13, E=6.06) for the control speaking condition. The mean value for the production of the SG−VF was 10.50 stuttered syllables (SD=9.67, SE=3.42), approximately a 55% reduction of stuttered syllables. The perception of SG +VF had a mean stuttering frequency of 5.38 stuttered syllables (SD=6.84, SE=2.42), respectively a 77% reduction of stuttered syllables. The EG +VF had a mean stuttering frequency of 6.13 stuttering syllables (SD=7.59, SE=2.68), approximately a 73% reduction of stuttered syllables. Finally, for SG+EG+VF the mean value of stuttering frequencies was 5.63 stuttered syllables (SD=5.61, SE=1.98), approximately a 76% reduction of stuttered syllables.
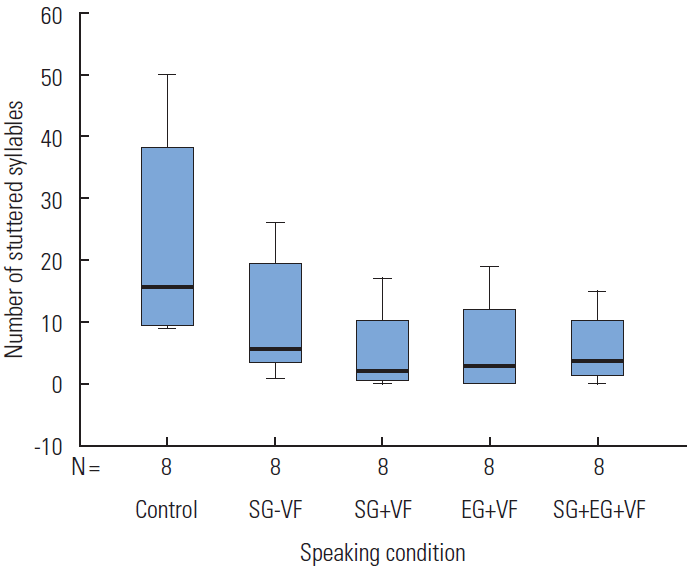
Minimum/maximum, inter-quartile, and median values for the Control Speaking Condition (Control), Self-Generated Prime without Visual Feedback (SG –VF), Self-Generated Prime with Visual Feedback (SG +VF), Externally-Generated Prime with Visual Feedback (EG +VF), and Self-Generated and Externally-Generated Prime with Visual Feedback (SG+EG +VF).
Due to the variance of overt stuttering severity within the small sample used in this study, a square root transformation was performed on the data before analysis, resulting in a more symmetrical and normalized distribution [20,31]. Using this transformed data, a one factor repeated measure analysis of variance (ANOVA) revealed a main effect of gestural priming on stuttering frequency [F(4,28)=11.890, Greenhouse-Geisser p=.004, η2=.629]. Bonferroni post hoc comparisons reveal a statistically significant difference between the control and SG−VF, SG+VF, SG+EG+VF, experimental speaking conditions (p<.001, p=0.012, and p=0.014, respectively).
Intrajudge and interjudge reliability compared there-analysis of 10% of the speech samples, chosen at random, with the original analysis of the data. Relative to stuttering frequency, an intrajudge syllable by syllable agreement was 0.93, as indexed by Cohen’s kappa, [32]. A second trained research assistant and the principal investigator both recalculated this 10% of the speech sample, chosen at random, (as described in previous research) [19] and found the stuttering frequency interjudge syllable-by-syllable agreement was 0.89, as indexed by Cohen’s kappa, [32]. Kappa values exceeding 0.75 suggest an excellent agreement beyond chance [31].
DISCUSSION
While mainstream psychological and speech-motor stuttering research paradigms can likely provide an explanation for these data [1,2], the following discussion will focus on the emerging neurological stuttering research paradigm, given the recent neurological and genetic developments in the literature [3–10]. Although existing research supports the activation of the mirror neuron system model relative to the enhancement of fluent speech in those who stutter [20,25], results from this study supports these data while using another novel (i.e., oral priming) stimulus and methods. In particular, these data confirm that the perception and production of initiatory gestures are not significantly different relative to efficacy of fluency enhancement; however, these data parallel previous findings in that the combination of self- and externally generated initiatory gestures trends toward significantly more efficacious fluency enhancement relative to either production or perception, alone [20]. These data, although utilizing oral gesturing rather than manual gesturing, are congruent with previous manual gesturing data in that both the production and perception of silent initiatory gestures significantly enhance fluency [17,20,25].
While these data continue to support the mirror neuron system as a theoretical model for the enhancement of fluency, little research has been done regarding the role of action understanding with respect to the enhancement of fluency in those who stutter. The existing literature and data suggest that mirror neuron systems may bypass certain higher order neural processes associated with stuttering, including the processes associated with speech and language [20,25,34,35]. These data applies to stuttering in that the primitive response of mirror neuron networks may enable an individual who stutters to fluently initiate speech via a primitive lower order network, and bypassing the activation higher order linguistic networks where the neural circuitry associated with stuttering is speculated to occur [20,35].
Coupled with existing research, these data support the supposition that stuttering behaviors are not the central pathology, but rather that stuttering behaviors in and of themselves are lower order compensations to higher order neural processing errors. This interpretation has been previously cited [10,20], with data suggesting that stuttering behaviors may be a form of endogenous gestural priming that the body is producing, thereby activating lower order primitive neural networks as an attempt to bypass the processing errors associated with stuttering and thus compensate for the pathology occurring at a central level [10,20,25]. Succinctly stated, stuttering behaviors may be a natural compensatory reaction to bypass a block in higher order linguistic-motor processing via a primitive lower order network. Accordingly, the compensatory nature of stuttering behaviors serve as gestural primes in an attempt to self-generate fluent speech [10,20,25,35]. This provides a novel insight into the role of stuttering behaviors themselves and supports that there is a genetic neural substrate associated with stuttering. Anecdotally, all but one participant in this study commented that the silent oral opening gesture used in this study was essentially identical to that of secondary stuttering behaviors.
Future research and clinical application
We believe this novel view delineating the central pathology (i.e., higher order genetic/neurological) from compensatory stuttering (i.e., primary and secondary stuttering behaviors) is crucial to the development of the science of stuttering. Due to the: (a) compensatory nature of stuttering behaviors, the (b) genetic and neurological substrate relative to the core pathology of the disorder, and (c) data such as these which link perception and production (and thereby the mirror neuron systems hypothesis) relative to robust fluency enhancement, we propose that the treatment of stuttering ought likewise evolve and adapt to reflect these developments in the science of stuttering. Treatments need to target the neural pathology, or at the very least, work with the neural and behavioral compensations (i.e., primary stuttering behaviors), as opposed to trying to suppress them. Additionally, future research in new treatment alternatives that integrate behavioral, prosthetic and pharmaceutical options is warranted, as they may better address the underlying core pathology of the disorder, and help optimize lower order activations or other behavioral compensations via multi-sensory initiatory priming via production (volitional stuttering) or perception (prosthetic speech feedback) of a SSS. While this exploratory research provides further evidence relative to the use of AUMN as a neurological substrate for the science and treatment of stuttering, future research may consider increasing the participant sample size, as a means to better delineate the relationships between stuttering behaviors, fluency enhancement, and activation of mirror neuron networks.