INTRODUCTION
The touchscreen interface has become very popular for personal mobile devices, including smartphones, tablets, and personal desktops because of the efficient and direct interaction with the display. Because of the rapid growth in touch technology, users with motor control disabilities also have been encouraged strongly to interact with this touch technology in work, healthcare settings, and service sectors, such as grocery stores, airports, and financial institutions [1].
Users with motor control disabilities may exhibit different touchscreen performance and physical demands compared to non-disabled users. A previous study showed that disabled groups had significantly greater numbers of trials with misses and errors, and took longer to complete digit entry tasks on a touch interface compared to non-disabled users [2]. Another study found that participants with gross motor control disabilities had greater impulses and longer dwell times during a tapping task compared to non-disabled participants [3]. In addition, participants with motor control disability showed greater perceived workload in a data entry task than did a control group, and the workload was higher particularly when oriented parallel to the touch screen compared to oriented to the front [4]. These findings indicated that disabled users exhibited lower touch performance and increased physical demands during touch screen interactions than did healthy users.
Not only motor control disabilities, but cognitive deficits as well could affect usersŌĆÖ touch characteristics. ParkinsonŌĆÖs disease (PD) patients revealed decreased brain activation when completing cognitive tasks and could not use oxygenation effectively in the frontal lobe region compared to healthy participants [5]. In this study, individuals with PD showed no significant changes in brain activation across 4 different cognitive tasks (1-the easiest to 4-the most difficult tasks). Other studies have revealed that basal ganglia dysfunction in PD patients may be associated with cognitive deficits in attention, working memory, reasoning, and executive functioning [6,7], and that PD patientsŌĆÖ cognitive function might affect their touch characteristics. Because touchscreen use requires hand gesture interactions, PD patients could have higher executive function demands with a touchscreen interface compared to the traditional desktop configuration (mouse and keyboard). To our knowledge, no evidence has shown the way touch technology influences PD patientsŌĆÖ cognitive function and touch performance.
Thus, the objective of this study was to investigate the effect of disability (PD patients vs. healthy participants) and desktop conditions (mouse vs. touchscreen) on usersŌĆÖ cognitive and motor function, and task performance. The first hypothesis was that patients with PD have lower cognitive function compared to healthy elderly subjects while using a mouse and touchscreen. The second hypothesis was that using a touchscreen device increases functional demands compared to mouse use.
METHODS
Participants
Six healthy older individuals with no history of neurogenic disorders and 6 PD patients were recruited for this study. ParticipantsŌĆÖ demographic information was collected and their anthropometric information is shown in Table 1. All participants had an auditory test and the Hohen-Yahr or Unified Parkinson Disease Rating Scale (UPDRS) scores for participants with PD were collected. The PD participantsŌĆÖ current medications were restricted to Carbidopa/Levodopa (Sinemet), Stalevo, Requip, and Azilect. The PD patients did not have tremor in their right hands. None of the participants had a history of drug or alcohol abuse. Participants signed a written informed consent approved by Northern Illinois UniversityŌĆÖs IRB before they participated in the research.
Instrumentation
Both traditional desktop and touchscreen conditions were provided in the workstation setup. The traditional desktop condition included a two-button external mouse and a 23-inch display. No keyboard and mouse were provided in the touchscreen condition, and users were required to use the 23-inch touchscreen display and perform a clicking task by finger tapping.
Functional near infrared spectroscopy (fNIR) was used to measure prefrontal cortex activity. The fNIR band (fNIR 400 system) included four IR light sources and ten detectors that were placed bilaterally on the subjectsŌĆÖ foreheads according to the international (10ŌĆō20) procedure for EEG electrode placement using the F7, Fp1, Fp2, and F8 (left and right hemispheres) positions, and were secured with a flexible headband. For motor function, kinematic data of the head/neck, and the movement of the hand and elbow were quantified with reflective markers at a sampling rate of 100 Hz using the Optitrack motion capture system (NaturalPoint
, Inc., Corvallis, OR). Six reflective markers were attached on the: 1) C7 spinous process; 2) right elbow; 3) right tragus; 4) right canthus; 5) knuckle of the right index finger, and 6) right side of the touchscreen.
The raw three-dimensional positional data were filtered initially with a digital, zero-phase 4th-order Butterworth filter at a cutoff frequency of 6 Hz (Motive, Optitrack, Natural Point, OR) and the elbow and hand travel velocities, neck flexion angle, head displacement, and gaze distance and angle were computed using biomechanics analysis software (Visual 3D, C-Motion Inc., Germantown, MD).
Testing procedure
The entire experiment required approximately 1.5 hour per participant, including reading and signing the consent and instrumenting the participants. Participants practiced the cognitive task before the main experiment and were instructed to perform it as quickly and accurately as possible. The spatial priming task was used in the Psychology Experiment Building Language (PEBL) Test battery to assess the immediate attention level in computer interaction [8,9]. This required participants to respond to a stimulus (target blue square) in a 3├Ś3 grid. Before the target square was presented, a visual cue (yellow square) that was presented in random grid locations flashed briefly, while there was no visual cue in some trials. The task in the touchscreen condition involved reaching and tapping the random stimulus using the index finger of the right hand. In the mouse condition, participants used the mouse to move the cursor and click the random stimulus. The trial duration was up to 2 minutes depending on each participantŌĆÖs performance. The order of the desktop conditions was counterbalanced to minimize the carryover effect.
Data analysis
In processing the fNIR data, the relative concentrations of the mean oxygenation values in the prefrontal cortex, including BrodmannŌĆÖs areas 9, 10, 45, and 46, were analyzed for each task. Baseline cerebral oxygenation levels (four in total) were obtained for each participant during the test to normalize values. Cognitive optical brain imaging (COBI) studio software was used to collect brain activation data. For each baseline, the participants were instructed to close their eyes for 30 seconds before each task, and fNIR data were collected continuously during each task. The normalized oxygenated hemoglobin (nHbo) and normalized total hemoglobin (nHbt) were calculated by subtracting the baseline from the task data.
For motor function, the elbow travel velocity was calculated as the finite difference in the distance between the elbow and C7 spinous process [10]. The hand travel velocity was computed similarly as the distance from the knuckle of the right index finger to the C7 spinous process. The neck flexion angle was calculated between the vertical line and the line from the tragus to the C7 spinous process. Head displacement was the horizontal distance from the C7 spinous process to the tragus [11]. Gaze distance was the distance between the canthus and the center of the display, and gaze angle was measured between a horizontal line and the line from the canthus to the center of the display. The median values (50th percentile) of the measures were summarized. For the cognitive test, the mean reaction time (ms) and proportion of correct responses (accuracy: %) were measured.
Statistical analysis
The dependent variablesŌĆÖ normality was assessed first using the Shapiro-WilkŌĆÖs W test. Because elbow and hand travel velocity and reaction time were not distributed normally, they were transformed using the log and Johnson transformations. Thereafter, a mixed factor two-way ANOVA was conducted in SPSS v. 24 (IBM Corporation, Armonk, NY). The desktop condition (within-subject factor) and disability (between-subject factor) were set as fixed effects, and the participant was set as a random effect. Given the non-normality of the accuracy variable, the non-parametric Friedman test was conducted; alpha was set at p<0.05.
RESULTS
fNIR analysis
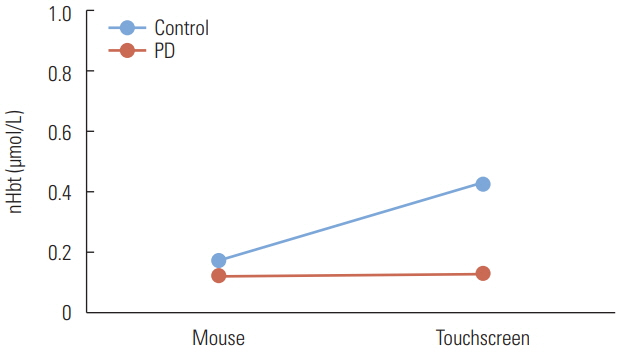
Table 2 provides the means and standard errors of nHbo and nHbt. The nHbo did not differ significantly between the desktop conditions (p=0.99) and disability (p=0.79), and there was no interaction effect (p=0.80). The desktop condition (p=0.28) and disability (p=0.51) did not affect the nHbt significantly, and there was no interaction effect (p=0.31). However, the control group showed a higher mean nHbt (0.31 ╬╝mol/L) than did the PD participants (0.13 ╬╝mol/L), and touchscreen use required a higher mean nHbt (0.29 ╬╝mol/L) than did mouse use (0.15 ╬╝mol/L).
Motor function analysis
Table 3 summarizes the means and standard errors of the motor function variables in the disability and desktop conditions. Touchscreen use required significantly higher elbow travel velocity (p=0.001) and hand travel velocity (p=0.03) than did mouse use. With respect to the postural variables, participants showed significantly more forward head displacement (p< 0.001) and a longer gaze distance (p=0.03) while using the mouse compared to the touchscreen. The gaze angle was significantly higher in touchscreen than in mouse use (p=0.01). Disability did not affect any motor function variables significantly, and there were no significant two-way interactions. Nevertheless, PD patients showed a higher neck flexion angle (71.2┬░) than did the control group (58.4┬░).
Task performance
Table 4 shows the means and standard errors of the task performance variables. The reaction time did not vary significantly in the desktop condition (p=0.96) and disability (p=0.06), and no interaction effect was found (p=0.40). PD patients exhibited a longer reaction time to complete the task (1,000 ms) than did the control group (796 ms). The desktop condition (p=0.66) and disability (p=0.66) did not influence accuracy significantly.
DISCUSSION AND CONCLUSIONS
This study investigated the effect of desktop condition (mouse vs. touchscreen) and disability (PD patients vs. healthy controls) on frontal lobe activity, motor function, and task performance. In addressing our previous hypotheses, the concentrations of oxygenated hemoglobin (nHbo) and total oxygenation (nHbt) did not differ significantly between PD patients and healthy participants, which did not support our first hypothesis. However, we observed a trend in which PD patients had much lower nHbt than did the control group in the two desktop conditions. In addition, touchscreen use did not increase functional demand significantly compared to mouse use, except the elbow/hand travel velocity, which supported our second hypothesis in part.
There were no significant differences in nHbo and nHbt between the PD patients and control participants, and this was consistent in both desktop conditions. This lack of significance could be related to the high variability in the fNIR measures and the limited sample size, both of which reduced the statistical power. Although it was not significant, PD patients had lower nHbo (0.27 ╬╝mol/L) than did control participants (0.34 ╬╝mol/L), which might indicate that the PD patients used oxygenation less effectively to complete tasks than did the control participants. In addition, the PD patients had much lower nHbt values (0.13 ╬╝mol/L) compared to the control participants (0.31 ╬╝mol/L), and this difference was considerably greater in the touchscreen than in the mouse condition (Figure 1). Unlike the control participants, PD patients did not reveal significant changes in brain activation between the mouse and touchscreen tasks. This finding is similar to that in the previous study that showed no significant differences in brain activation among PD participants across cognitive load tasks with four different levels of difficulty [5]. This might suggest that PD participants exhibited a consistent lack of brain activation regardless of desktop conditions.
The lower prefrontal cortex activation on the part of PD patients could be associated with cognitive deficits in executive functions [5], which might explain in part why PD patients had slower response times during the attentional cuing task (1,000 ms) than did the control group (796 ms). This difference in prefrontal lobe activity between PD patients and the control group might be greater while using more complex user interfaces, as previous studies have shown that the difference between PD patientsŌĆÖ and healthy participantsŌĆÖ frontal lobe oxygenation levels was lowest in the easiest cognitive load task and highest in the most difficult task [5,12].
The motor function results showed that both PD patients and control participants moved their hands approximately 5 times faster while using the touchscreen than the mouse. Because mouse use allowed participants to stabilize their right hands on the mouse, the slower hand movement was not surprising. This result is consistent with a previous study that found that a full touch task (touchscreen only) required an elbow travel velocity approximately twice as fast than did a no touch task (using a keyboard and mouse) in a desktop PC setting [10]. They also reported that the full touch task required longer durations of floating arm postures compared to the no touch task, and this longer period without arm support could exert greater demands on the shoulder muscles while using a touchscreen. PD patients exhibited lower hand travel velocity than did the control group, and the difference was greater in the touchscreen setting (PD: 0.09 cm/s; control: 0.13 cm/s). This could explain in part why PD patients had slower responses during cognitive tasks (1,000 ms) than did the controls (796 ms), which is consistent with the previous study that reported that PD patients took longer than normal to respond to a visual stimulus [13].
Mouse use caused more forward head displacement and a longer gaze distance compared to touchscreen use. During mouse use, both participant groups held their heads approximately 1cm farther forward compared to during touchscreen use. Anterior head displacement during mouse use could be related to the increased moment-arm of the head, which would increase the gravitational load on the neck [14,15]. While using a touchscreen, participants approached the display to touch the screen efficiently with their index fingers, during which they had an approximately 6cm shorter gaze distance than while using a mouse. It has been shown that a closer viewing distance is associated with increased risk of visual fatigue [16], and placing the eyes at least 50 cm from the display has been recommended to prevent visual strain [17, 18]. Although touchscreen use was associated with a shorter gaze distance (58 cm) than was mouse use (65 cm), the gaze distance was only slightly greater than 50 cm, and therefore the risk of visual fatigue was not substantial in this study.
Touchscreen use required an approximately 3┬░ greater gaze angle than did mouse use, which was consistent with a previous study that found that gaze angle in the touch condition was significantly higher than in the no touch condition [10]. This greater gaze angle could be associated with increased muscle demand in the neck and shoulder regions [10,19]. However, the gaze angle (16┬░) in the touchscreen task here was only slightly higher than the 15┬░ ANSI/HFES 2007 recommends [17], so the gaze angle itself would not cause substantial physical demands on the neck in the touchscreen condition.
Although it was not strictly significant (p=0.07), PD patients tended to exhibit greater neck flexion (71┬░) than did control participants (58┬░) under both conditions. Antecollis is a common postural deformity in PD patients that results in excessive forward flexion of the neck [20,21], which could be related to our finding. These flexed neck postures are associated with increased muscle demands in the neck and the development of neck pain [15,22]. Thus, PD patients could exhibit greater functional demands on the neck compared to healthy users during both mouse and touchscreen tasks.
Although this laboratory study was designed and controlled carefully, it had several limitations. First, the small sample size (total of 12 participants) reduced the statistical power of our measures. However, we recruited PD patients who had no tremor in their right hands and control participants who were comparable in age to those in the PD group explicitly to reduce excessive variability among them. Second, PD patients with tremor were not evaluated in this study, and Parkinson tremor is a common movement disorder that is observed often in the hands or feet [23]. It would be worthwhile to investigate the way PD patients with tremor interact differently with touchscreen devices than a control group or PD patients with no tremor. Lastly, only an attentional cuing task (simple user interface) was conducted in this study. In reality, users could experience more complex computer user interfaces while using a touchscreen. Because different levels of cognitive load tasks were associated with PD patientsŌĆÖ frontal lobe activities [5], future studies could study the way complex cognitive tasks influence the cognitive and motor function of PD patients while using touchscreen devices.
In conclusion, PD patients with no tremor and healthy participants had no significant differences in frontal lobe activities while using a mouse and touchscreen, although PD patients exhibited consistently lower total oxygenation levels compared to control participants in both desktop conditions. Touchscreen use required significantly higher elbow/hand travel velocity than did mouse use, as expected. While engaged in the attentional cuing task, PD patients showed lower hand travel velocity, longer response times, and greater neck flexion compared to the control group. These results suggest that PD patients exhibit lower cognitive and motor function than do healthy users while interacting with both a mouse and touchscreen.